European Authorised Representative
European Authorised Representative

Background knowledge
In order to better protect consumers and the environment in the EU and to ensure the traceability of medical devices, the European legislator requires product´s manufacturers to affix CE marking and labeling with their names and addresses on the product, which they want to place on the EU market; For manufacturer who located outside the European Economic Area (including the EU and EFTA), his product must be accompanied bay the name and contact addresses of the manufacturer and of his European Authorized Representative (EAR).
In order to improve the overall efficiency of market surveillance, the European Commission requires all member states to meet the low legal requirements. The EU Commission is working with Customs, the relevant manufacturers, European Authorised Representatives, importers and distributors to set up a complete traceability system for products.
Definition of European Authorized Representative
European Authorised Representative is a company that is explicitly designate by a manufacturer who places a product on its own name and located outside the EEA (including the EU and EFTA). This company can act on behalf of the non-European manufacturer and perform his duties and responsibilities in accordance with relevant EU directives and law.The new directive requires that the European Authorised Representative must to be located within the European Economic Area and have a commercial registered address (some EU countries also require the company registration number or EU VAT number from the European authorized representative).The governments and competent authorities of the EEA member states may anytime directly verify by the European Authorised Representative whether the non-EU manufacturer has fulfilled actually the required of the relevant EU directives and laws.The manufacturer´s general sales representative (eg authorized dealer), whether or not located within the European Economic Area, should not be confused with the European Authorised Representative by the new directive.Although the European Authorised Representative, on behalf of the non-EU manufacturer, can perform the duties and responsibilities required by the relevant EU Directives and laws, the manufacturer remains the primary responsibility for taking over.Without the manufacturer's consent, the EU authorized representative is not allowed to modify the products manufactured by the non-EU manufacturer, even if to make the non-compliant product to comply with the requirements of the EU Product Directive.
The European Authorised Representative should keep the following documents:
1.Declaration of conformity (DOC),
2.Copies of labels, packaging and instructions for use (in all required languages from the countries where the products are placed on the market),
3.(relevant) certificates and evidence from the notified body),
4. Post-market surveillance processes and data, vigilance reports und complaints,processes and data,
5.relevant technical documentation of market surveillance conducted by the member states,
6.relevant clinical data /announcements,
7.Detailed information of all distributors/ suppliers that bring the product to market.,
8.Incident reporting as well as the correct measures taken
The responsibilities of European Authorised Representative include:
a)as an authorized representative of the manufacturer, responsible for contacting the competent supervisory authorities in the member states within the EU to handle the incidents, complaints, adverse events and recalls of medical devices;
b)preserve technical documentation of the manufacturer, when the supervisory authorities have question, contact the manufacturer in order to answer the questions and communicate with supervisory authorities;
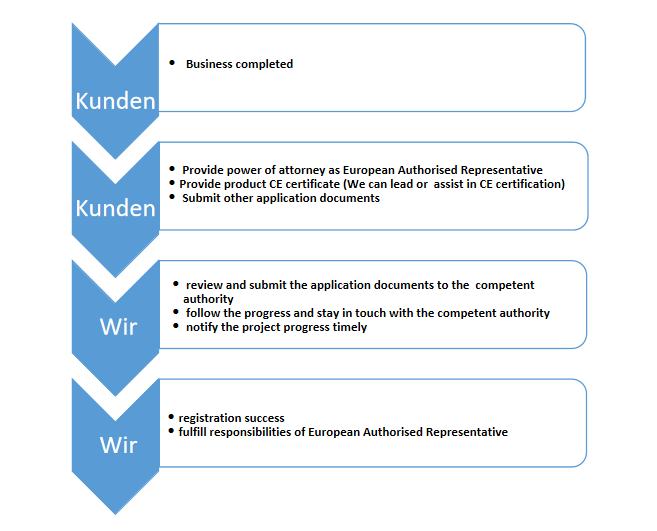
c)medical devices registration on behalf of the manufacturer in the European Union;
d)application for a sales permit on behalf of the manufacturer in the European Union.
We provide the services such as European Authorized Representative (EAR, EC Rep) and related certification and wish you success in Europe!
Download
Contact us
Tri Rad Medical GmbH
 Tiergartenstraße 32
Tiergartenstraße 32
 01219 Dresden Germany
01219 Dresden Germany
 +49 (0) 351 – 2086 4877
+49 (0) 351 – 2086 4877
 +49 (0) 351 – 8627 8683
+49 (0) 351 – 8627 8683